Table Of Content
Such guidelines rather serve an educational purpose of making researchers aware of possible pitfalls and biases before the experimental conduct. There are resources to assist investigators in designing rigorous protocols and identify sources of bias. Cross-referencing to experimental reporting guidelines and checklists (e.g. ARRIVE (NC3Rs 2018a), the NIH guidelines (NIH 2018a) and the Nature reporting of animal studies checklist (Nature 2013)) can be informative and helpful when planning an experimental protocol. However, it is important to bear in mind that these are primarily designed for reporting purposes and are not specifically designed for use in assisting with experimental design.
Exploratory and Confirmatory Research
A single group is studied at a single point in time after some treatment that is presumed to have caused change. The carefully studied single instance is compared to general expectations of what the case would have looked like had the treatment not occurred and to other events casually observed. The EDA can then also generate a randomization sequence or compile a report of the planned experiment that can, e.g. be part of a preregistration of the experimental protocol.
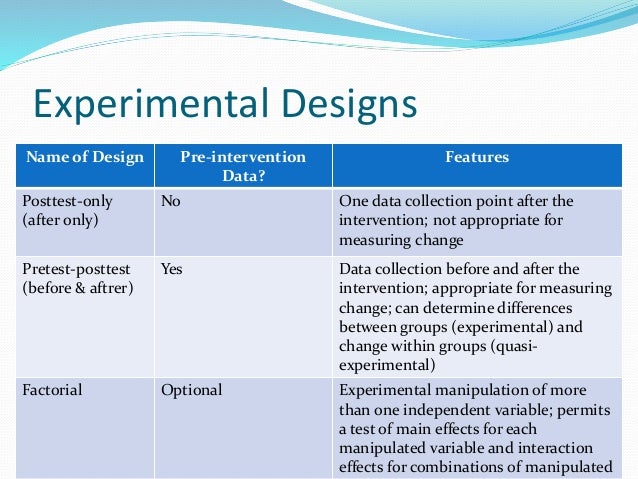
One-shot case study design
A single case is observed at two time points, one before the treatment and one after the treatment. Changes in the outcome of interest are presumed to be the result of the intervention or treatment. In a pre-experimental design, there is typically only one group of subjects, and this group is measured or observed both before and after an intervention or treatment. Characteristics of pre-experimental design include its ability to determine the significance of treatment even before the true experiment is performed.
Advantages of Pre-experimental Designs
People naturally impose their own assumptions about causes and needed actions, and these constitute, in the crudest sense, theories of the problem and the solution. A formal process of mapping objectives, evidence, and formal theories of behavior and social change to the design of coherent, practical interventions includes guidelines for how to assess evidence and theories (Bartholomew et al., 2006). The mapping of theory helps the decision maker fill gaps in the evidence and consider how the evidence from a distant source and a particular population may or may not apply to the local setting, circumstances, and population.
Many argue that only external costs should be considered when making decisions about whether policy interventions are warranted. In conclusion, pre-experimental design, while limited in its ability to provide strong evidence of causality, plays a crucial role in exploratory research. It presents a simplified and cost-effective approach to experimentation that is especially useful when resources are limited or when the goal is to explore a new area of study. However, the inherent limitations of pre-experimental designs necessitate caution in interpreting their results.
Chapter 5.2 Pre-Experimental Design
Even research projects that do not involve administering necessary medications or treatments may limit the researcher’s ability to conduct a classic experiment. Any pretest–posttest design may be subject to plausible versions of one or more of these threats to its level of certainty (internal validity) depending on the specific research context, making it very difficult to conclude that a causal effect of the treatment has occurred. Once again, following Campbell’s tradition, the strategy of adding design elements to address specific plausible threats can help provide more confidence that the treatment rather than other confounding factors has had the desired effect. For example, replicating the pretest–posttest design at different times with different cohorts of individuals can help rule out history effects, while taking several pretest measures to estimate the maturation trend in the absence of treatment can help rule out history.
In this way, they could classify patients in experimental and comparison groups without dictating state policy or telling people where to live. Another approach to assign participants to experimental and comparison groups in a quasi-experimental design is through matching. Researchers should think about what variables are important in their study, particularly demographic variables or attributes that might impact their dependent variable. When this is done at the beginning of an experiment, the matched pair is split—with one participant going to the experimental group and the other to the control group. In contrast, an ex post facto control group is when a researcher matches individuals after the intervention is administered to some participants.
Outcome bias in self-evaluations: Quasi-experimental field evidence from Swiss driving license exams - ScienceDirect.com
Outcome bias in self-evaluations: Quasi-experimental field evidence from Swiss driving license exams.
Posted: Tue, 09 Aug 2022 19:12:39 GMT [source]
In this way, they could classify patients in experimental and comparison groups without affecting policy or telling people where to live. The RD design is viewed as one of the strongest alternatives to the RCT from both Campbell’s (Cook, 2008; Shadish et al., 2002; Trochim, 1984) and Rubin’s (Imbens and Lemiuex, 2008; Rubin, 1977) perspectives. However, it introduces two new challenges to causal inference that do not characterize the RCT. First, it is assumed that the functional form of the relationship between the quantitative assignment variable and the outcome is properly modeled.

Pre-/Posttest Designs
The original Head Start program included not only its well-known educational program, but also basic health services to children (e.g., nutrition supplements and education, immunization, screening). In addition to positive effects on educational achievement, Ludwig and Miller found results demonstrating lower mortality rates in children aged 5 to 9 from diseases addressed by the program (e.g., measles, anemia, diabetes). Pre-experiments offer few advantages since it is often difficult or impossible to rule out alternative explanations. The nearly insurmountable threats to their validity are clearly the most important disadvantage of pre-experimental research designs. Despite these limitations, pre-experimental designs can serve as valuable starting points in exploratory research, laying the groundwork for more rigorous experimental designs in the future. Another characteristic of pre-experimental design is the absence of random assignment.
Reporting of the precise details of bias reduction methods is often scanty, and therefore accurate assessment of the precise method and rigour of such procedures is challenging. Moreover, those papers that do not report one bias-reducing method, e.g. randomisation, also tend to not report other bias-reducing methods, e.g. blinding and sample size calculation, suggesting that there could be interactions between these methods. Measurements are taken to assess the results; these are recorded as outcome measures (also known as dependent variable). The primary outcome measure should be identified in the planning stage of the experiment and stated in the protocol; it is the outcome of greatest importance, which will answer the main experimental question. The number of animals in the experiment is determined by the power needed to detect a difference in the primary outcome measure.
There are many different quasi-experimental designs in addition to the nonequivalent comparison group design described earlier. Describing all of them is beyond the scope of this textbook, but one more design is worth mentioning. Additionally, multiple observations afterwards allow the researcher to see whether the intervention had lasting effects on participants. With respect to units, participants (people or small clusters of people), times, settings, or outcome measures may serve as the units of analysis. Research in public health and medicine commonly assigns treatments to individual (or small groups of) participants.
When true experiments are not possible, researchers often use quasi-experimental designs. In pre-experimental designs, researchers observe or measure subjects without manipulating variables or controlling conditions. Often, these designs lack certain elements of a true experiment, such as random assignment, control groups, or pretest measurements, making it difficult to determine causality. Researchers that wish to conduct a true experiment in medical science or social work may be required to deny necessary treatment to clients, which is violates professional ethics.
Nonetheless, this design can be useful for exploratory studies aimed at testing a measures or the feasibility of further study. When true experiments and quasi-experiments are not possible, researchers may turn to a pre-experimental design (Campbell & Stanley, 1963). [4] Pre-experimental designs are called such because they often happen before a true experiment is conducted. Often, researchers want to see if their interventions will have an effect on a small group of people before they seek funding and dedicate time to conduct a true experiment.
If you cannot manage the ethical norms along with your research study, your research objectives and validity could be questioned. Usually, researchers miss out on checking if their hypothesis is logical to be tested. If your research design does not have basic assumptions or postulates, then it is fundamentally flawed and you need to rework on your research framework.

No comments:
Post a Comment